
If you have PSC and are struggling with itching, you may be eligible to join the VISTAS Study and help explore an investigational medicine, volixibat, which may help manage itch related to PSC. The study will consist of a screening period, treatment period, and follow-up period—with the option to participate from the comfort of your home.
The study requires following specific guidelines and keeping track of your symptoms and any side effects. Your commitment is essential to ensuring accurate results and could help in developing more treatment options for the PSC community.
Volixibat is an investigational medicine being studied to help with itching in people with PSC. It works by blocking a protein in the small intestine that normally returns bile acids to the liver. Instead, the bile acids leave the body through stool. This may lower bile acid levels in the blood, which could help protect the liver from damage. Volixibat mostly stays in the gut and does not enter the bloodstream. It can be taken with most other PSC medicines.

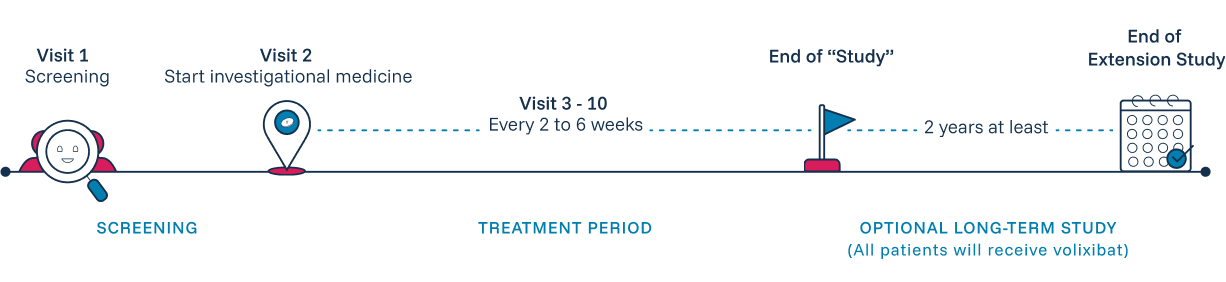
The VISTAS Study will enroll patients ages 12 and over diagnosed with PSC to see if volixibat can safely and effectively reduce itching caused by PSC. Participants will be randomly assigned to take either volixibat or a placebo (a treatment with no active ingredient) twice a day by mouth. This study is blinded, meaning neither the participants nor the study doctor and staff know who is getting volixibat and who is getting the placebo.
The study aims to see if volixibat is safe and effective in lowering bile acid levels, which may help improve itching and other symptoms of PSC. The study is designed to be flexible, allowing some or all visits to be done from the comfort of your home. Participants will need to record their itch level once a day, which could take less than two minutes.
Participants may have the option to continue in a long-term extension of the study where they will be guaranteed to receive volixibat.
Participating in the VISTAS Study is designed to be as convenient and supportive as possible. The blinded study will last at least 36 weeks, during which the study team will conduct health assessments, ask questions, and perform study-related tests to monitor overall health, side effects, and changes in your quality of life. Some or all visits can be done from the comfort of your home.
Throughout the study, a dedicated medical team will monitor your health and support you every step of the way. If you have a primary doctor, we strongly recommend letting them know you’re considering this study so they can support your decision.

We offer the convenient option of allowing you to participate in our studies from the comfort of your own home.
A healthcare professional will monitor you through a mix of telemedicine and home visits. The study doctor’s role is limited to the clinical study.
While in the study, you will continue your routine medical care and see your regular doctors for check-ups and prescriptions.



Additional study requirements and exclusions apply. A study representative will discuss these with you during the screening period.
An investigational medication is a drug that has not been approved by the Food and Drug Administration (FDA) or other health organizations. However, it can still be given to participants in a clinical study as part of research. A clinical study tests how safe and effective an investigational medication is in volunteers. Every investigational medication must go through clinical studies.
Agreeing to join a clinical trial is entirely up to you. Even if you sign the informed consent form, you’ll be free to leave the trial at any time.
Qualified participants may receive compensation for time and travel-related expenses (including long-distance travel). Study-related medications, procedures, and examinations are provided at no cost to study participants.
If you agree to participate, you will be expected to follow the rules and instructions to the best of your ability. If you are not able to follow these rules and instructions, you may be asked to withdraw from the study.
In order to help provide maximum protection for your health, the study will be under the direct supervision of the study doctor and will be conducted by trained personnel. You will need to provide all information about your current and past health (medical history) at the Screening Visit and each Follow-Up Visit, including participation in any other research studies. This information is needed to help protect your health.
If you have a primary doctor, it is strongly recommended that you inform him/her of your interest in participating in this research study.
Cholestatic pruritus is the itching sensation caused by various liver conditions. PSC is one of the main causes of cholestatic pruritus.2
1. National Center for Biotechnology Information. PubChem Compound Summary for CID 24987688, Volixibat. Accessed Feb. 12, 2025. https://pubchem.ncbi.nlm.nih.gov/compound/Volixibat. 2. Zakharia K, Tabibian A, Lindor KD, Tabibian JH. Complications, symptoms, quality of life and pregnancy in cholestatic liver disease. Liver Int. 2018;38: 399–411. https://doi.org/10.1111/liv.13591.

We offer the convenient option of allowing you to participate in our studies from the comfort of your own home. A healthcare professional will monitor you through a mix of telemedicine and home visits. The study doctor’s role is limited to the clinical study. While in the study, you will continue your routine medical care and see your regular doctors for check-ups and prescriptions.