Primary biliary cholangitis (PBC) is a progressive inflammatory liver disease that causes the small bile ducts in the liver to become injured and inflamed—and eventually destroyed. When there are no bile ducts, bile builds up and causes liver damage, ultimately requiring a liver transplant for many patients.
While PBC affects mostly women, men may also develop this condition. The exact cause is unknown, but it may be linked with issues in the immune system. People with a family member who has PBC may also have a higher chance of developing it.
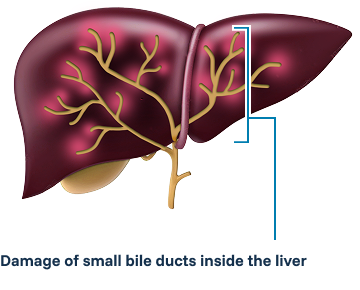
Doctors may diagnose PBC based on your medical and family history, a physical exam, and the results of medical tests. Medical tests used to diagnose PBC may include blood tests, imaging tests, and liver biopsy.








A clinical study, also called a clinical trial, is a research test to see if an investigational medicine is safe and effective in human volunteers. It compares the investigational medicine to a placebo (a treatment with no active ingredients). These medicines have not been approved by the FDA (Food and Drug Administration) or other health organizations. However, they can still be given to people as part of the study. Participants play a vital role in advancing medicine for current and future generations.

Mirum Pharmaceuticals, Inc. is a biopharmaceutical company conducting clinical studies to investigate a potential treatment for itch associated with PSC and PBC in pediatric and adult individuals.
For more information about Mirum, please visit MirumPharma.com.
1. Mayo Clinic. Primary biliary cholangitis. Accessed February 10, 2025. https://www.mayoclinic.org/diseases-conditions/primary-biliary-cholangitis/symptoms-causes/syc-20376874. 2. National Institute of Diabetes and Digestive and Kidney Diseases. Primary biliary cholangitis. National Institutes of Health. Accessed February 12, 2025. https://www.niddk.nih.gov/health-information/liver-disease/primary-biliary-cholangitis. 3. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: Emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035. 4. World Health Organization. Clinical trials. WHO. Accessed February 12, 2025. https://www.who.int/health-topics/clinical-trials#tab=tab_1.

We offer the convenient option of allowing you to participate in our studies from the comfort of your own home. A healthcare professional will monitor you through a mix of telemedicine and home visits. The study doctor’s role is limited to the clinical study. While in the study, you will continue your routine medical care and see your regular doctors for check-ups and prescriptions.