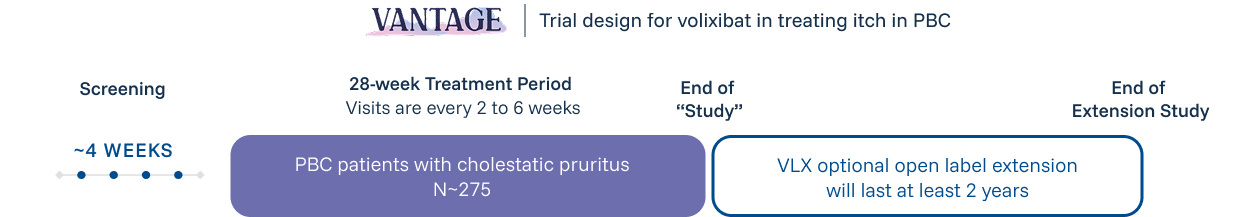
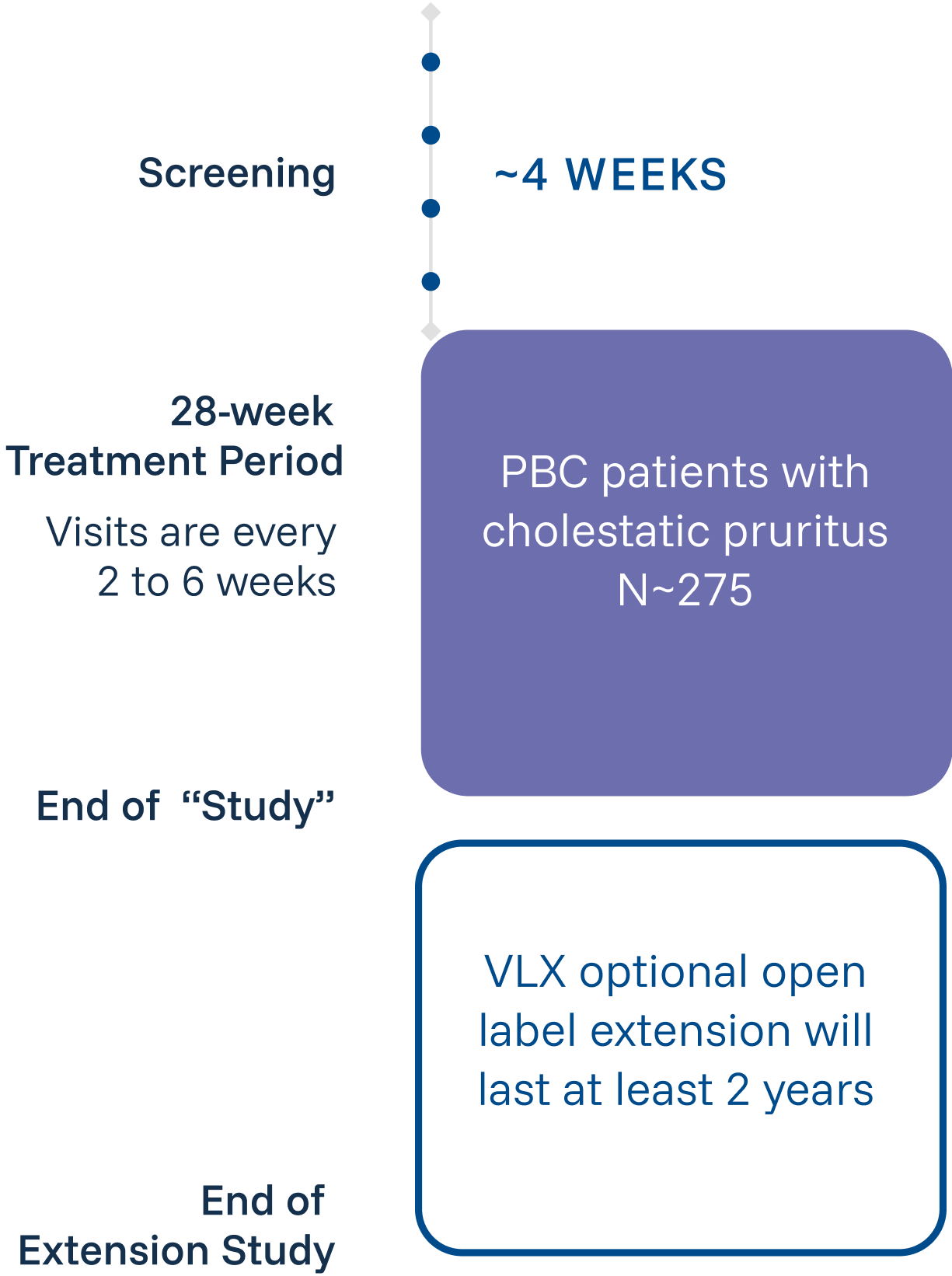
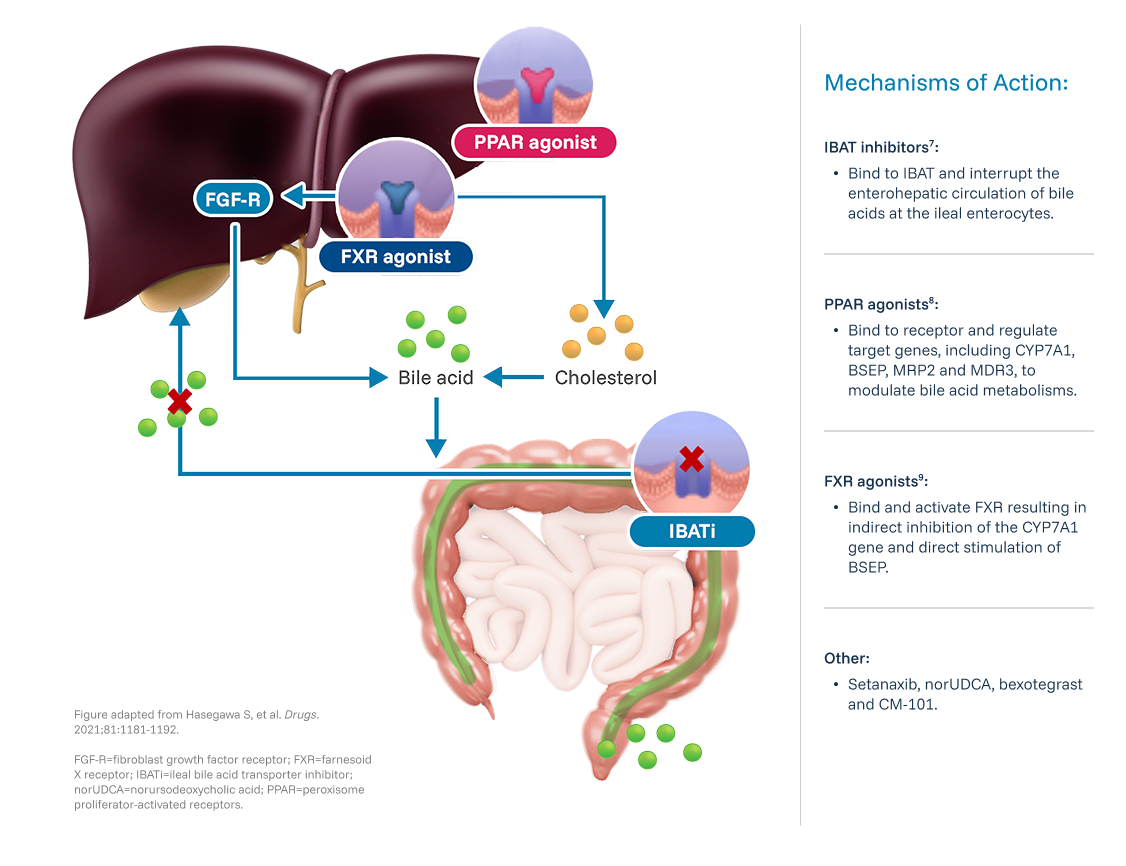
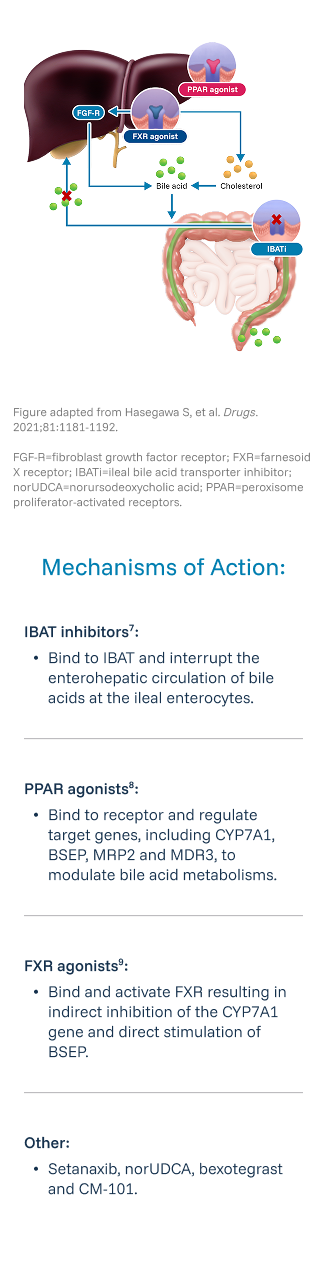
Review the following criteria to determine if your patient qualifies for the VANTAGE Study.
Ages Eligible for Study: 18 Years and older
Sexes Eligible for Study: All
Accepts Healthy Volunteers: No
Inclusion Criteria:
- Provide signed informed consent at the screening visit as well as comply with all study visits and requirements through the end of the study.
- Male or female, age ≥18 years at the screening visit.
- Confirmed diagnosis of PBC in line with the AASLD guidelines.
- UDCA and anti-pruritic medication use will be allowed if meeting additional criteria.
- Qualified pruritus associated with PBC as assessed by Adult ItchRO.
Exclusion Criteria:
- Pruritus associated with an etiology other than PBC.
- Evidence or clinical suspicion of decompensated cirrhosis or a history of decompensation events.
- Current symptomatic cholelithiasis or inflammatory gallbladder disease.
- History of small bowel surgery/resection impacting the terminal ileum that may disrupt the enterohepatic circulation.
- Evidence, history, or suspicion of other liver diseases; PBC patients with AIH are not excluded.
- History of liver transplantation.